In September 2020, Pfizer’s CEO, Albert Bourla, assured the public that ‘we will develop our vaccine using the highest ethical standards’. So let’s take a look at the recent history of Pfizer’s ‘ethical standards’ by which their COVID-19 ‘vaccine’ has been developed.
In 1992, Pfizer agreed to pay between $165 million and $215 million to settle lawsuits arising from the fracturing of its Bjork-Shiley Convexo-Concave heart valve, which by 2012 had resulted in 663 deaths.
In 1996, Pfizer conducted an unapproved clinical trial on 200 Nigerian children with its experimental anti-meningitis drug, Trovafloxacin, without parental consent, causing the death of 11 children from kidney failure and leaving dozens more disabled.
In 2011, Pfizer paid just $700,000 to four families who had lost a child, and set up a $35 million fund for the disabled. This cover-up was the basis to the John Le Carré book and film, The Constant Gardener.
In 2004, Pfizer’s subsidiary, Warner-Lambert, was fined $430 million to resolve criminal charges and civil liabilities for fraudulently promoting its epilepsy drug, Neurontin, paying and bribing doctors to prescribe it for uses not approved by the FDA.

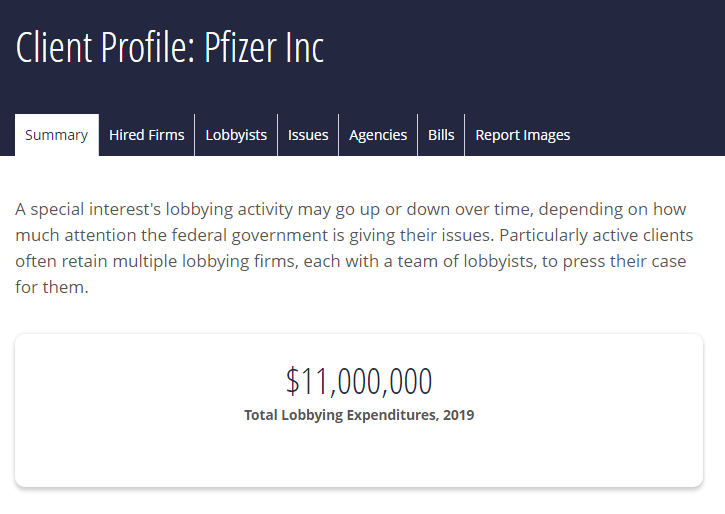
In 2009, Pfizer spent $25.8 million lobbying Congressional lawmakers and federal agencies like the Department of Health and Human Services. Its expenditure on lobbying between 2006 and 2014 came to $89.89 million. In 2019 it spent $11 million on lobbying.
In 2009, Pfizer paid the largest ever health care fraud settlement and criminal fine, $2.3 billion to avoid criminal and civil liability for fraudulently marketing its anti-inflammatory drug, Bextra, which had been refused FDA approval for safety concerns.
In 2009, Pfizer paid $750 million to settle 35,000 claims that its drug, Rezulin, was responsible for 63 deaths and dozens of liver failures. In 1999, a senior epidemiologist at the FDA said Rezulin was ‘one of the most dangerous drugs on the market’.
In 2010, Pfizer was ordered to pay $142.1 million in damages for violating a federal anti-racketeering law by its fraudulent sale and marketing of Neurontin for uses not approved by the FDA, including for migraines and bi-polar disorder.
In 2010, Pfizer admitted that, in the last 6 months of 2009 alone, it had paid $20 million to 4,500 doctors in the US for consulting and speaking on its behalf, and $15.3 million to 250 academic medical centres for clinical trials.
In 2012, Pfizer paid $45 million to settle charges of bribing doctors and other health-care professionals employed by foreign governments in order to win business.
The Chief of the Foreign Corrupt Practices Act Unit said: ‘Pfizer subsidiaries in several countries had bribery so entwined in their sales culture that they offered points and bonus programs to improperly reward foreign officials who proved to be their best customers’.
By 2012, Pfizer had paid $1.226 billion to settle claims by nearly 10,000 women that its hormone replacement therapy drug, Prempro, caused breast cancer.
In 2013, Pfizer agreed to pay $55 million to settle criminal charges of failing to warn patients and doctors about the risks of kidney disease, kidney injury, kidney failure and acute interstitial nephritis caused by its proton pump inhibitor, Protonix.
In 2013, Pfizer set aside $288 million to settle claims by 2,700 people that its drug, Chantix, caused suicidal thoughts and severe psychological disorders. The FDA determined that Chantix is probably associated with a higher risk of heart attack.
In 2013, Pfizer absolved itself of claims that its antidepressant, Effexor, caused congenital heart defects in the children of pregnant woman by arguing that the prescribing obstetrician was responsible for advising the patient about the medication’s use.
In 2014, Pfizer paid a further $325 million to settle a lawsuit brought by health-care benefit providers who claimed the company marketed its epilepsy drug, Neurontin, for purposes unapproved by the FDA.
In 2014, Pfizer paid $35 million to settle a law suit accusing its subsidiary of promoting the kidney transplant drug, Rapamune, for unapproved uses, including bribing doctors to prescribe it to patients.

In 2016, Pfizer was fined a record £84.2 million for overcharging the NHS for its anti-epilepsy drug, Phenytoin, by 2,600 per cent (from £2.83 to £67.50 a capsule), increasing the cost to UK taxpayers from £2 million in 2012 to about £50 million in 2013.
In May 2018, Pfizer had 6,000 lawsuits pending against claims that its testosterone replacement therapy products cause strokes, heart attacks, pulmonary embolism and deep vein thrombosis, and were marketed at healthy men for uses not approved by the FDA.
As of 22 September, 2021, the MHRS has received 330,983 reports of adverse drug reactions to the Pfizer ‘vaccine’, including 544 deaths within 7 days of injection, from 117,297 people. The Government estimates that between 2% and 10% of ADRs are reported.
For more on this pharmaceutical company, whose vast profits alone have allowed it to escape criminal prosecution, please read our report.
Pfizer expects to generate $33.5 billion in COVID-19 ‘vaccine’ sales in 2021. The company generated $9.2 billion in vaccine sales in the second quarter of 2021 alone, compared to $1.2 billion during the same period last year.

Adapted from Architects For Social Housing
















One thought on “Pfizer’s history of “high ethical standards””